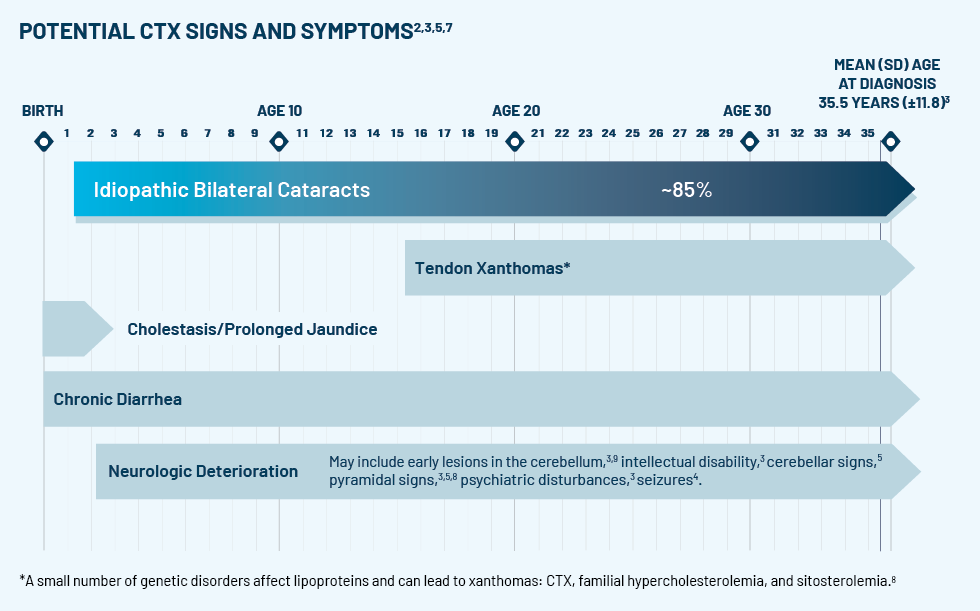
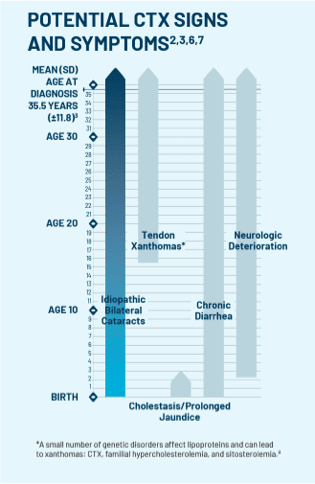
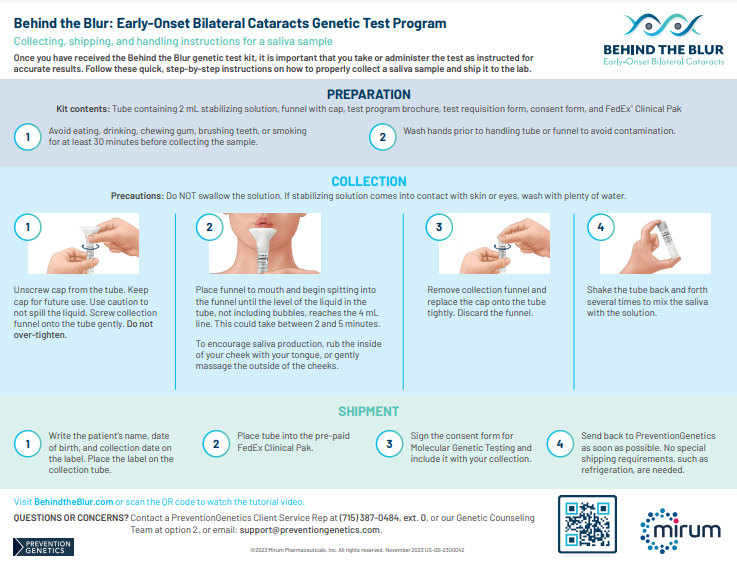
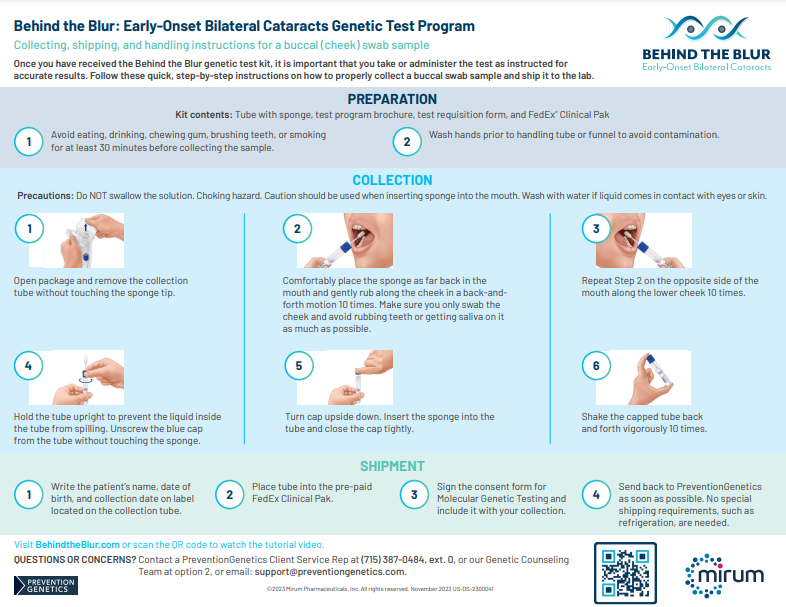
If the test comes back positive for CTX, treat cataracts, continue to monitor, and refer your patient to a neurologist or metabolic geneticist for CTX management.
References: 1. Raymond GV, Schiffmann R. Cerebrotendinous xanthomatosis: the rare “treatable” disease you never consider. Neurology. 2019;92(2):61-62. 2. Lorincz MT, Rainier S, Thomas D, Fink JK. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol. 2005;62(9):1459-1463. 3. Mignarri A, Gallus GN, Dotti MT, Federico A. A suspicion index for early diagnosis and treatment of cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2014;37(3):421-429. 4. Gallus GN, Dotti M, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27(2):143-149. 5. Verrips A, van Engelen BG, Wevers RA, et al. Presence of diarrhea and absence of tendon xanthomas in patients with cerebrotendinous xanthomatosis. Arch Neurol. 2000;57(4):520-524. 6. Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. Juvenile cataract morphology in 3 siblings not yet diagnosed with cerebrotendinous xanthomatosis. Ophthalmology. 2013;120(5):956-960. 7. Clayton PT, Verrips A, Sistermans E, Mann A, Mieli-Vergani G, Wevers R. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2002;25(6):501-513. 8. Farpour H, Mahloudji M. Familial cerebrotendinous xanthomatosis. Report of a new family and review of the literature. Arch Neurol. 1975;32(4):223-225. 9. Fraidakis MJ. Psychiatric manifestations in cerebrotendinous xanthomatosis. Transl Psychiatry. 2013;e302. 10. Verrips A, Hoefsloot LH, Steenbergen GCH, et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123(pt 5):908-919. 11. Federico A, Dotti MT, Gallus GN. Cerebrotendinous xanthomatosis. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle. 1993-2020. Updated April 14, 2016. Accessed October 26, 2020. http://www.ncbi.nlm.nih.gov/books/NBK1409/ 12. Freedman SF, Brennand C, Chiang J, et al. Prevalence of cerebrotendinous xanthomatosis among patients diagnosed with acquired juvenile-onset idiopathic bilateral cataracts. JAMA Ophthalmol. 2019;137(11):1312-1316. 13. PreventionGenetics Panel Data.